Photoelectron Spectroscopy
Accessing chemical properties of interfaces via X-ray photoelectron spectroscopy

X-ray photoelectron spectroscopy (XPS)
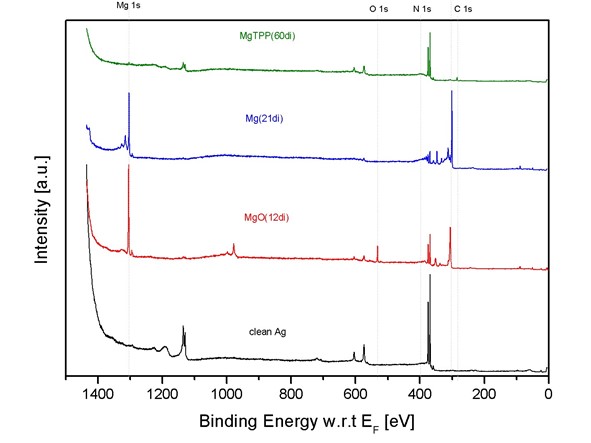
X-ray photoelectronic spectroscopy (XPS) is a surface analytical tool based on the photoelectric effect that measures the energies of electrons in atoms and molecules. As this binding energy of the electrons in their shells is element specific the measurement yields a quantitative chemical analysis of a sample surface. Furthermore, small shifts of the elemental binding energies give information of the chemical state and the bonding environment.
To measure an XPS spectrum the sample is irradiated with monochromatic X-rays and the emitted photoelectrons are detected as a function of their kinetic energy by an electron spectrometer (Fig. 1). This can be expressed in the formula:
hυ-BE=KE+ΦSpec,
where the energy of the X-ray photon (hν) minus the electron binding energy (BE) is equal to the kinetic energy (KE) of the emitted photoelectron plus the spectrometer work function (φSpec). A typical XPS spectrum is shown below (Fig. 2), where the number of electrons detected at a specific binding energy is plotted. Each element produces a set of characteristic XPS peaks.
Within the surface science group Graz three ultrahigh vacuum XPS systems are available, equipped with a dual anode X-ray source combined with a hemispherical electron energy analyser. The systems include facilities for fast entry sample transfer, manipulation, Ar+ ion cleaning and heating.